Supercritical CO2 extraction of essential oils and its advantages and disadvantages
Landlord:17600603718 time:12-12 10:18 Click:2126 Reply:0 Collection
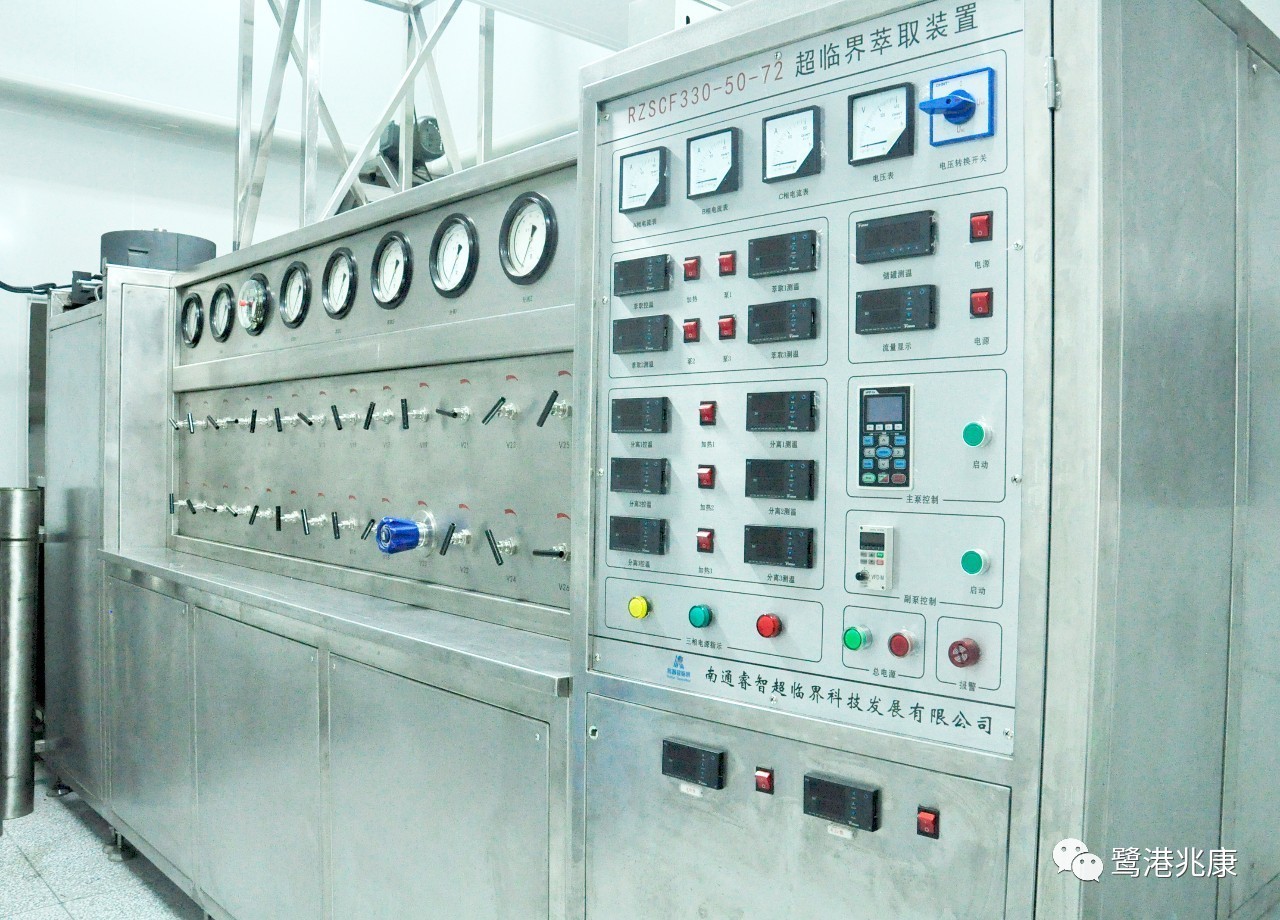
The CO2 (carbon dioxide) extraction method for essential oils is often referred to as CO2 extraction or abbreviation for supercritical. In short, CO2 extraction is by pressurized carbon dioxide until it becomes a critical point for liquid extraction. The liquid carbon dioxide then acts as a solvent to obtain natural plant matter and volatile oil content, and carbon dioxide dissolved in the liquid. The CO2 is then brought back to natural pressurization, the carbon dioxide is evaporated back to its gaseous state and the remainder is the resulting oil.
This set of “high pressure, low temperature” extraction methods began in the 1980s. The instruments and equipment are very complicated and expensive. The essential oil is labeled “CO2 Extraction”. This extraction is carried out in a closed reaction tank, and the time required is very short, in just a few minutes (distillation takes at least 1 hour).

advantage:
Compared to solvent extraction, this extraction method does not leave any solvent residue.
Carbon dioxide extracts are generally thicker than distilled essential oils and often smell closer to the natural grass. The carbon dioxide extract has been said to contain additional components that are extracted by steam distillation from the same plant. This seems to make sense because carbon dioxide extracts are usually thicker oils and tend to have a more comprehensive aroma.
This method is a highly specialized extraction method. Because the solvent evaporates, the essential oil contains no solvent impurities and the separation is thorough. The extracted molecules can be larger than the classification of the distillation method. Here is a case to explain, the distillation method is to bring out aromatic molecules through steam, but the larger molecules are not extracted, such as the diterpene molecules in the happy sage, which is already the limit, that is to say 20 The extraction of terpenes of carbon atoms by distillation has been rarely seen.
Disadvantages:
Of course, some people also question that carbon dioxide is an acid gas, so this type of extraction will more or less destroy the chemical structure in the essential oil.
Although the plant essential oil extracted in this way will be very close to the original aromatic composition in the plant, it is a perfect extraction method. However, the equipment used in this extraction method is not only large but also very expensive. Taking it to extract essential oils often takes several years to balance costs. Until today, the price of essential oils extracted with supercritical carbon dioxide is still very expensive.
Surprisingly, it is not necessarily close to nature. In the case of ginger, the scent of ginger essential oil extracted with supercritical carbon dioxide is naturally better than the distilled ginger essential oil, but it is also easy to irritate sensitive skin. In addition to being expensive, there is a shortcoming in the essential oils extracted from supercritical carbon dioxide, which is that few manufacturers are willing to provide and are not easy to buy.
